Reactions of Unsaturated Acceptors: Methodology and Applications to Functionalization via Radical Pathways
Michela Maria Marchese1, Olivier Riant2
1 Institute of Condensed Matterand Nanoscience (IMCN), Molecules, Solids and Reactivity (MOST),Place Louis Pasteur 1 bteL4.01.02, 1348 Louvain-la-Neuve, Belgium.
2 Institute of Condensed Matterand Nanoscience (IMCN), Molecules, Solids and Reactivity (MOST),Place Louis Pasteur 1 bteL4.01.02, 1348 Louvain-la-Neuve, Belgium.
michela.marchese@uclouvain.be
The introduction of fluorine in a molecule has remarkable effects, for example its biological behaviour can be deeply changed. Usually the introduction of this moiety affects pKa, lipophilicity, toxicity, and metabolic stability. -CF3 and -CF2 are the most common units employed. In literature, there are less examples with -CF2 and for this reason, we decided to develop a methodology for the introduction of this group into N-heterocycle. The idea was mainly based on previous study1 where lactones are synthetized through a radical pathway using copper as a catalyst and Togni reagent as fluorine source. In our investigation we decided to use different alkenyl amine with different length of the aliphatic chain to form six- and five-member rings. IF2C2O2Et is the fluorine source we decide to use. The general reaction is in schema 1.
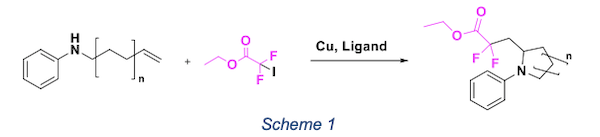
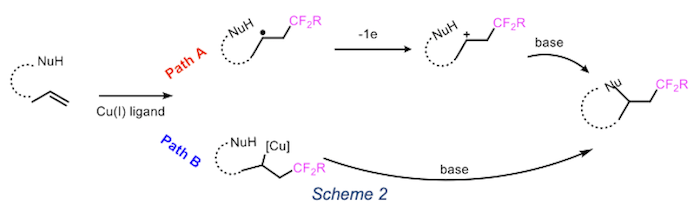
Our strategy is based on the ability of copper to act as radical initiator. This atom transfer radical addition (ATRA) is feasible pathway for the formation of carbon–carbon bonds starting from alkyl halides and alkenes. According to the literature2,3, the ring formation can follow two possible mechanism reported in scheme 2. Currently we are using this methodology for the formation od functionalized pyrrolidine and piperidine. These scaffolds are common moiety in natural product and exhibit a variety of biological effects.
References:
1. Zhu, R. & Buchwald, S. L. Versatile Enantioselective Synthesis of Functionalized Lactones via Copper-Catalyzed Radical Oxyfunctionalization of Alkenes. J. Am. Chem. Soc. 137, 8069–8077 (2015).
2. Yu, Q. & Ma, S. Copper-catalyzed cyclic oxytrifluoromethylation of 2,3-allenoic acids to trifluoromethylated butenolides. Chem. - A Eur. J. 19, 13304–13308 (2013).
3. Lin, J. S. et al. Efficient copper-catalyzed direct intramolecular aminotrifluoromethylation of unactivated alkenes with diverse nitrogen-based nucleophiles. Chem. - A Eur. J. 20, 1332–1340 (2014).
