Studying the Mechanical Reversibility of a Click-Chemistry Linkage by AFM-Based Single-Molecule Force Spectroscopy
Valentin Foidart, Narangerel Ganbaatar, and Anne-Sophie Duwez
Nanochemistry and Molecular Systems, Department of Chemistry, University of Liege, Belgium
vfoidart@uliege.be
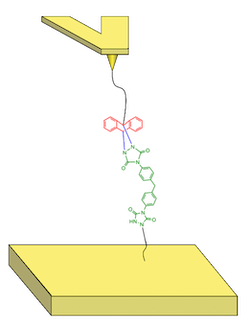
The experimental system is composed of a gold-coated tip and surface. A polycaprolactone polymer is grafted to the tip thanks to a gold-sulphur interaction and linked to the anthracene on the other side. The MDI-TAD molecule is linked to a citronellol which is anchored on the surface with a gold-sulphur bond. Contrary to what is expected for a classical covalent bond, our study showed that the TAD-anthracene click bond appears to break at much lower forces.
- Mondal, P., Jana, G., Behera, P. K., Chattaraj, P.K., Singha, N. K. A New Healable Polymer Material Based on Ultrafast Diels-Alder ‘Click’ Chemistry Using Triazolinedione and Fluorescent Anthracyl Derivatives: A Mechanistic Approach. Polym. Chem. 2019, 10 (37), 5070-5079.
- Billiet, S., De Bruycker, K., Driessen, F., Goossens, H., Van Speybroeck, V., Winne, J. M., Du Prez, F. E, Nat. Chem. 2014 (6), 815.
- De Bruycker, K., Billiet, S., Houck, H. A., Chattopadhyay, S. Winne, J. M., Du Prez, F. E. Triazolinediones as Highly Enabling Synthetic Tools. Chem. Rev. 2016, 116 (6), 3919-3974.
