Valorization of biomass-derived glycerol over acidic hafnium-doped silica nanotubes – playing with sol-gel kinetic
L. Soumoy1, C. Célis1, D. Debecker2, C. Arprile1*
1 Laboratory of Applied Materials Chemistry (CMA), Unit of Nanomaterials Chemistry (CNANO), Department of Chemistry, University of Namur
2 Université catholique de Louvain (UCLouvain), Institute of Condensed Matter and Nanosciences (IMCN), 1348 Louvain-la-Neuve, Belgium
*carmela.aprile@unamur.be
In the current context of environmental concerns, growing efforts are constantly dedicated to develop efficient processes based on new feedstocks while reducing energy consumption or waste generation. It is not surprising that heterogenous catalysts play a significant role in this transition, mainly due to the possibility of recycling them in multiple cycles. In the large family of heterogenous catalysts, the class of mesoporous silica-based materials open the door to a plethora of applications. The chemical and physical stability, the mild conditions of synthesis or the possibility to functionalize them, allow designing materials responding to a wide range of needs. In particular, metallosilicates showed promising catalytic activities in the context of biomass valorisation and in particular in the conversion of triose sugar to lactate, hexose to furfural or in the valorisation of glycerol.
In this work, we combined the innovative morphology of hollow silica nanotubes with the intrinsic properties of hafnium atoms to design, in a straightforward one-pot procedure, an acid catalyst able to valorise the biodiesel by-product – glycerol – into solketal (Figure 1). [1,2] The influence of various synthesis parameters as the nature of Hf precursor ligand or the concentration of the reaction mixture were investigated. Through extensive characterization via inter alia HR-TEM or XPS, we evidenced the impact of the studied parameters on hafnium coordination environment. NH3-TPD measurements allowed to confirm that the incorporation of Hf in Si-nanotubes generates acid sites which are the catalytically active species for the target reaction. Since changes of the chemical environment of hafnium and/or different amount of acid sites were pointed out, all our Hf-doped silica nanotubes were tested in the conversion of glycerol into solketal to investigate their catalytic activities. The evolution of the turnover frequencies was highly related to the proper insertion of Hf in the nanotubes and to the amount of acid sites. Our best catalyst was proven to be stable but also recyclable thanks to a simple one-step reactivation procedure. Through the variation of catalytic tests conditions, it was possible to achieve yield approaching the total conversion of glycerol.
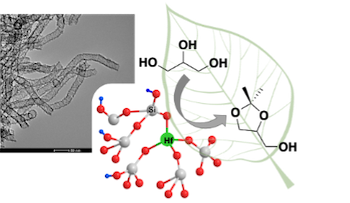
Figure 1. Hafnium-doped silica nanotubes catalysing the conversion of glycerol into solketal
- L. A. Bivona, Appl. Catal. B. 2019, 247, 182-190.
- L. Li, Green Chem. 2012, 14, 1611-1619
