Enriching active carbon with nitrogen groups: an alternative opening doors for thiosulfate leaching in gold mining
N. de la Torre, S. Hermans
IMCN-MOST, UCLouvain
sophie.hermans@uclouvain.be
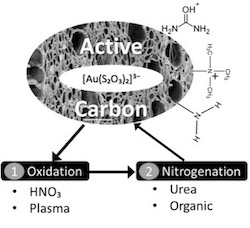
To enrich active carbon with nitrogen groups, the general strategy involves the solid’s oxidation followed by its nitrogenation. Active carbon could be successfully oxidized by either HNO₃ or air-humidified Glid-Arc plasma. This was confirmed by Boehm titrations, evidencing an increase of acidity in the oxidized solid from 8 to 160 mmol/100 g. Oxidized carbons are rich in carboxylic acids, that are essential for grafting nitrogen groups in the next steps. Two methods were tested for the samples nitrogenation: 1) urea method and 2) organic method 2. The urea method consisted in impregnating the oxidized solid with an urea saturated solution. The organic method involved multi-step reactions including acylation of the oxidized material and further contact with diamines to obtain either primary or quaternary amine sites.
XPS analysis on the urea-method produced carbon evidenced the increase of surface nitrogen from 1 at.% (non-treated carbon) to 5 at.%. The high-resolution nitrogen peak showed the presence of different nitrogen species including amine/ amides ( ̴ 400 eV). Regarding the organic-method produced carbon, XPS analysis showed an increase in the nitrogen content up to 5 at.% and different nitrogen groups (mainly amines) were also noted. Nitrogen groups were as well corroborated by FTIR (band ̴ 3200 cmˉ1) in all samples. Then, the nitrogen-rich carbons were contacted with a gold thiosulfate solution (10 ppm) to assess their performance. It was observed that while the non-functionalized carbon traps 20 % of gold from the solution, the functionalized ones accomplished: 68 % for urea impregnated carbons and 100 % recovery for primary and quaternary aminated carbons, which verifies the hypothesis that the presence of nitrogen groups enhances the gold thiosulfate adsorption capacity of the modified solid.
1. Aylmore, M. G., Muir, D. M. & Alymore, M. G. Thiosulfate Leaching of Gold--a Review. Miner. Eng. 14, 135–174 (2001).
2. Vidick, D. et al. Comparison of functionalized carbon nanofibers and multi-walled carbon nanotubes as supports for Fe-Co nanoparticles. J. Mater. Chem. A 1, 2050–2063 (2013).
